New ways to engage patient and other stakeholder in clinical trial planning
New ways to engage patient and other stakeholder in clinical trial planning
25 June 2019 Comments Off on New ways to engage patient and other stakeholder in clinical trial planningClinical trials are research studies that evaluate whether a medical treatment or a medical device is safe and effective for treating a disease. Clinical trials provide important information to patients, healthcare providers, policy makers to make evidence-based healthcare decisions and improve health outcomes. However, these stakeholders do not often participate in planning clinical trials to decide which questions should be answered and how the trials should be designed. As a result, clinical trials do not always generate information relevant to the needs of these stakeholders. The trials are conducted in controlled settings, which makes it difficult to translate the information generated to clinical practice in real-life settings. The impractical design of clinical trials also causes unnecessary burden for clinical trial participants, for example, long questionnaires, frequent clinic visits with long waiting time.
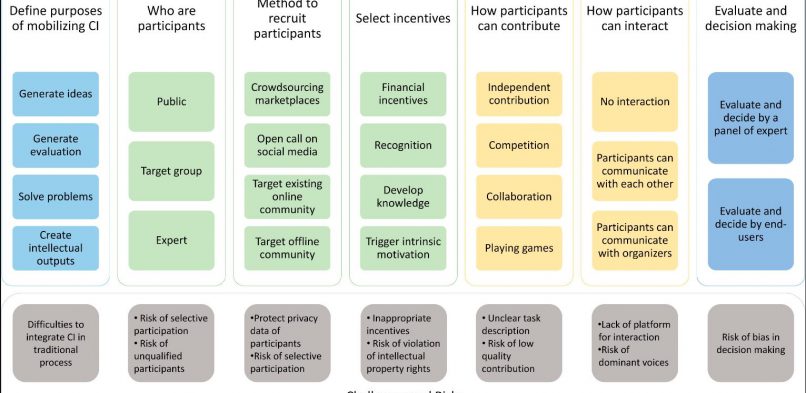
My PhD project looks for new methods to enable the participation of diverse stakeholders in planning clinical trials such as patients and public members, healthcare providers and policy makers. I am particularly interested in new methods which have emerged outside medical research such as crowdsourcing and open innovation. Crowdsourcing enables public members to contribute to science by completing an assignment. With the help from a large and diverse group of individuals, scientists can complete their experiments in an effective manner. Open innovation, which is often used in business, acknowledges that external stakeholders can contribute innovative ideas to develop products that meet the needs of consumers.
In healthcare, these two methods have been used to involve diverse stakeholders in research and intervention development. For example, Cochrane crowd is a project which involve thousands of participants who help medical researchers to classify a large amount of medical literature (1). Bill and Melinda Gates foundation launched a competition to public members to seek for new ideas of intervention to support caregivers in making decisions of vaccine utilisation (2).
In my research, I identified different ways of these new methods to involve stakeholders in research by looking at scientific literature across different research fields (see my publication here). I also talked to researchers who used these methods to understand the challenges that they faced, their tips and lessons learnt when involving stakeholders in their research (see my publication here). This information will help me to prepare for a practical application of using these new methods to involve patients and public members in planning clinical trials. My research will also compile good practice advice which will support researchers who wants to try new ways to bring the voices of diverse stakeholders in their research.
Why am I interested in this project? After graduating from pharmacy school, I joined a clinical trial unit in Vietnam and managed the implementation of several clinical trials. Although in Vietnam patient were not involved in planning clinical trials, through communication with patients and clinicians, we made several changes to trial protocols to improve experience of patients who participated in the trials such as reducing number of unnecessary blood tests. This experience helped me understand the values of patients’ opinions in clinical trial planning. This PhD project is an opportunity for me to find new ways to integrate effectively perspectives of patients and other stakeholders to increase research value.
by Van Nguyen Thu, MiRoR PhD Fellow at the University Paris Descartes & University of Liverpool
1) http://crowd.cochrane.org/index.html
2) https://www.openideo.com/challenge-briefs/gates-vaccine-innovation